The delivery of health and care services in Scotland is becoming increasingly reliant on healthcare IT. Whilst healthcare IT has the ability to deliver substantial benefits to patients, it can also introduce the potential for harm.
Why do a CSC?
• SG Digital Strategy and Data Strategy 2021
The Scottish digital health and care strategy has set out a commitment to develop and embed a standards-based approach to clinical and care safety cases for all major systems. In addition, it sets out a standard to ensure clinical safety of our systems is embedded throughout, noting that all software classed as a medical device must be compliant with current UK regulations.
Irrespective of these strategic commitments is the concept that a CSC is the "right thing to do" due to its potential to positively impact on the quality and safety of healthcare IT.
• Previous news stories of Healthcare IT going wrong
The ultimate aim of doing a CSC is to improve the safety and quality of healthcare IT. Numerous headlines over the years have demonstrated the real-world impact of healthcare IT failures, underscoring the critical need to ensure its safety.
• Clinical lens
A clinical safety approach differs from other risk management frameworks in that it focuses purely on potential patient harm. Financial, reputational or any other organisational risks are not considered as part of the clinical risk assessment.
• Prevent AEs occurring at a later date
Conducting a CSC promotes identification of risks/hazards in a clinical system before they lead to harm, allowing these to be addressed and therefore improving the reliability of the system and reducing adverse events. CSCs promote a positive safety culture and proactive risk assessment.
• Diagram
The below diagram shows the benefits of doing a CSC and the main outputs than can be produced.
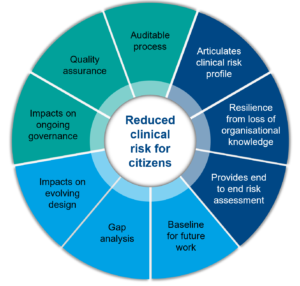
We undertake a comprehensive end to end clinical safety risk assessment as a large team. Members from our clinical safety case team work alongside experts from across the deploying programme to develop a shared understanding of the clinical safety approach, the digital solution itself, and drive out any uncertainty, ambiguity and assumptions.
Articulation of the clinical risk profile - our comprehensive risk assessment allows us to clearly articulate the profile of clinical risk a deploying programme is exposed to.
Resilience from loss of organisational knowledge - the documents that we collate and generate as part of a clinical safety case offers the programme a degree of resilience from a loss of organisational knowledge.
End to end clinical risk assessment - The main benefit of a clinical safety case is the potential to highlight clinical risks associated with deploying a digital solution, some of which may have been previously unknown. As part of the clinical safety risk assessment we identify hazards that could result in patient harm, the causes of these, and any existing or potential controls.
Baseline for future work/gap analysis/Impact on evolving design – a comprehensive end to end clinical risk assessment gives us the opportunity to identify hazards, causes and potential controls. Identifying areas that may benefit from further development can impact on the evolving design of the solution and feed improvement plans, ensuring that the clinical risk is reduced as low as reasonably possible.
Impact on governance - our comprehensive risk assessment identifies areas where the governance surrounding a digital solution may require review or improvement.
Assurance - our comprehensive risk assessment offers a level of quality and safety assurance for any regulatory scrutiny, should this occur.
Auditable process - our clinical safety process is fully auditable due to alignment with multiple international standards and a quality management system approach e.g. ISO 14971, 13485, 62304.