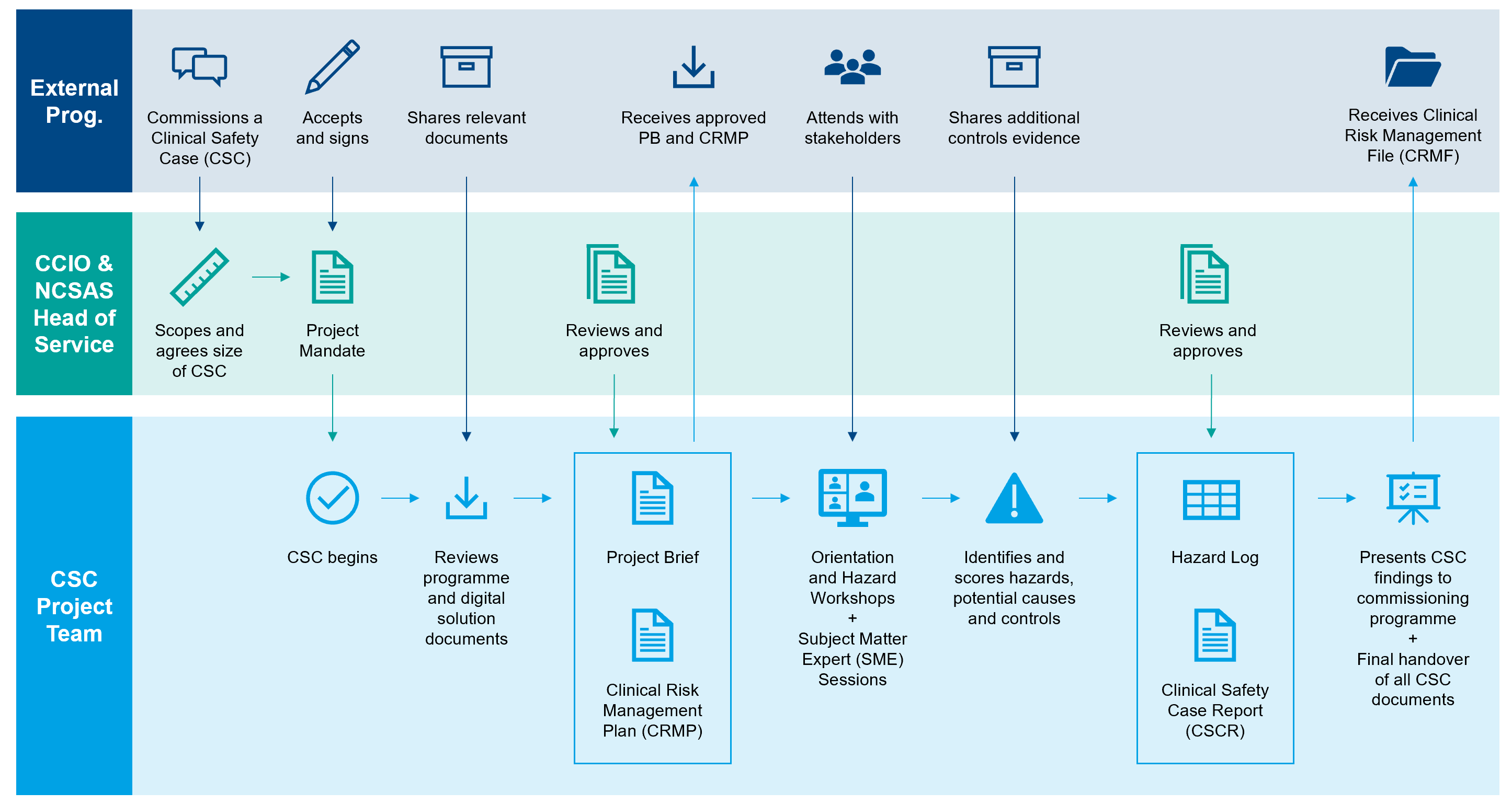
Below is a high level process flowchart for developing a Clinical Safety Case (CSC).
The external/commissioning programme is the customer that receives the CSC output in the Clinical Risk Management File (CRMF).
The Chief Clinical Informatics Officer (CCIO) and the Head of Service for National Clinical Safety Assurance Service (NCSAS) are accountable for signing off the content of the Clinical Safety Case Report (CSCR) and Hazard Log.
The CSC Project Team are responsible for delivering the CSC and associated products.