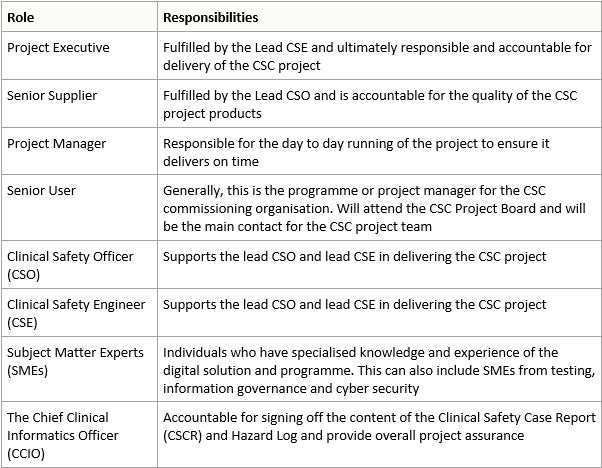
In producing a Clinical Safety Case (CSC) a lead Clinical Safety Officer (CSO) and a lead Clinical Safety Engineer (CSE) are assigned to the CSC.
The lead CSO and CSE are supported by other CSOs and CSEs, with a Project Manager overseeing the delivery of the CSC project.
Regular Project Board meetings are held with the Senior User to provide updates on progress and review any risks or issues that have been identified.
details of the responsibilities for each role are noted in the below table:
Throughout the CSC process, a good working relationship with the Senior User and the SMEs is essential to delivering a quality and valuable CSC. This is very much a 'team sport' and not something that can be delivered in isolation by the CSC project team.